Re:
"Not necessary for AGM and gel, they are explosion-proof."
SDA: My comments follow below.
No...and this is a dangerous myth. In many ways, VRLA's are less safe than regular flooded LA.
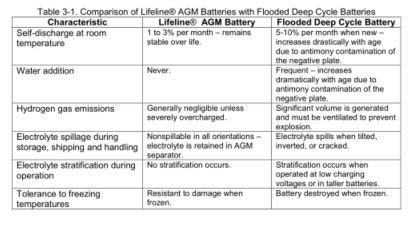
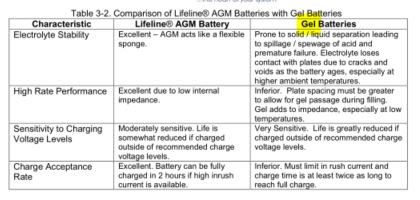
ALL lead-acid batteries make hydrogen when charged. Valve Regulated Lead Acid types (including liquid, AGM and Gel) keep hydrogen contained inside the battery
up to a point (typically 2psi). However, cell-wise degradation impacts all of these designs over time and results in overcharging of cells. Near their end-of-life, all of these batteries begin making make MORE hydrogen as cells fail. Too much hydrogen will overwhelm the hydrogen recapture mechanisms in VRLAs, causing them to release hydrogen above the 'regulated' threshold.
Whether it is 'contained' in the battery or not, hydrogen explodes.
SDA: Point taken, I should not have said "proof" should have said resistant. I didn't say SVRLAs don't need to be vented for hydrogen removal, just that they don't explode. I have never encountered a case of either battery type that has exploded. AGM's can be air-shipped BTW. Has anyone on the forum encountered an AGM or gel that has exploded?
Indeed, they can overheat, catch fire etc, however, it's unlikely anything other than a metallic case would do any good. I'd argue that batteries in boxes are more likely to suffer this fate because no one sees what's going on until it's critical. I have seen AGMs overheat and the cases melt and deform grossly, but they still did not leak.
The ABYC standard for storage...
10.7.2 Provision shall be made to contain incidental leakage and spillage of electrolyte.
NOTE: Consideration should be given to:
1. the type of battery installed (e.g. liquid electrolyte or immobilized electrolyte).
2. the boat in which the battery is installed (e.g. angles of heel for sailboats, and accelerations for powerboats).
In fact (taking a lesson from the data-center world), a combination of factors including the pervasive 'explosion-proof' myth, new failure modes that are unique to these batteries (thermal runaway), and 'fail in the dark' syndrome mean that these batteries -- which ABYC groups together as "immobilized electrolyte" types -- are just as dangerous or moreso than flooded types.
1) The explosion-proof myth. See:
LMGTFY
LMGTFY
2) Thermal runaway. One thing flooded Lead-Acid batteries
almost never do is CATCH FIRE. Thermal runaway is a failure mode that is associated with immobilized electrolyte batteries, and frequently results in a fire.
http://lmgtfy.com/?q="vrla"+"thermal runaway"+"fire"
SDA: No argument, however, in practice this simply isn't pervasive in marine applications. Perhaps more of an issue in data centers. Flooded batteries produce explosive hydrogen gas with every charge cycle, SVRLA's only do so in the event of a malfunction.
3) "Fail-in-the-dark" syndrome. These batteries all have one thing in common -- you can't check the electrolyte, so naturally you don't. Because these batteries are marketed as 'maintenance free' and because of the 'explosion proof' mythology, and because there is no way to test individual cells, people become careless. With carelessness comes a tendency to forget about them.
SDA: Agreed, but this occurs with flooded batteries as well, I routinely encounter dry flooded batteries. When SVRLA's are overcharged, in he vast majority of cases they simply vent dry out and lose capacity, at which point they are replaced (and the same thing often occurs again).
Thirty-odd years ago during my early days working in the data-center world, uninterruptible power supplies with various immobilized electrolyte batteries were taking the place of -48v telco grade flooded battery banks for keeping critical equipment running during power outages. It took the industry several years to learn the new failure modes. Even though the battery manufacturers were telling data center managers that these batteries would become a fire-hazard after three years or so, replacements were deferred anyway. See photos below.
Re:
"While full containment is not required by ABYC standards, my own rule for flooded batteries is a full box with lid for this very reason, they can explode."
Again, all these batteries can explode. A covered battery box will not save anyone from an exploding battery, it just creates more flying shrapnel and possibly more resulting damage.
SDA: I'm sorry, you are simply mistaken about this, and again your experience in the data center world may simply not apply to this industry. I've inspected the results of a number of battery explosions, and witnessed three first hand, all those in FRP or poly boxes were fully contained.
To the extent that the ventilation in the lid may be insufficient to clear all the hydrogen (or maybe you threw something on top of the cover box and blocked the vent), a covered battery box can increase the hydrogen concentration in the area of the terminals -- kaboom.
SDA: Agreed, and ABYC standards address this, calling for ventilation at the apex of every lid, purpose made battery boxes include this vent.
Lastly, because a covered battery box traps heat, the chances of a VRLA going into thermal runaway are increased.
SDA: Agreed, and I made that point in my post, boxes are of no value and may be harmful for SVRLA's. In the case of a flooded battery, full containment is desirable to capture leaking acid if nothing else, but also, in my first hand experience, to contain flying acid and battery parts in the event of an explosion, which is far more more likely in a flooded battery as a result of routine hydrogen production.
According to ABYC (E-10.7.7), there are two (and only two) reasons for requiring a battery box; (a) contain liquid electrolyte when it spews out of the vents and (b) protect the battery terminals from incidental short.
SDA: As noted above in E-10.7.2 a box may be used as containment. E-10.7.7 says nothing about "contain liquid electrolyte when it spews out of the vents". Here it is in its entirety...
10.7.7 To prevent accidental contact of the ungrounded battery connection to ground, each battery shall be protected so that metallic objects cannot come into contact with the ungrounded battery terminal and uninsulated cell straps. This may be accomplished by means such as:
10.7.7.1 covering the ungrounded battery terminal with a boot or non-conductive shield, or
10.7.7.2 installing the battery in a covered battery box, or
10.7.7.3 installing the battery in a compartment specially designed only for the battery(s).
NOTE: When batteries have both a stud and post arrangement, protection should preclude contact with any part of the terminal.
My advice: properly maintained and with a good charger, conventional flooded types last longer, cost less and do not present risk of thermal runaway. To the extent that they are carefully monitored at the individual cell level, I would strongly argue that regular flooded types are SAFER than VRLA's. I would avoid VRLAs unless there is some compelling need, for example you might want one in a waverunner...